NEWS
Color change is only device modification. Is a new 510k required? - Medical Device Academy
By A Mystery Man Writer

This article explains the process for determining if a color change and other material changes require a new 510k prior to implementing the change.
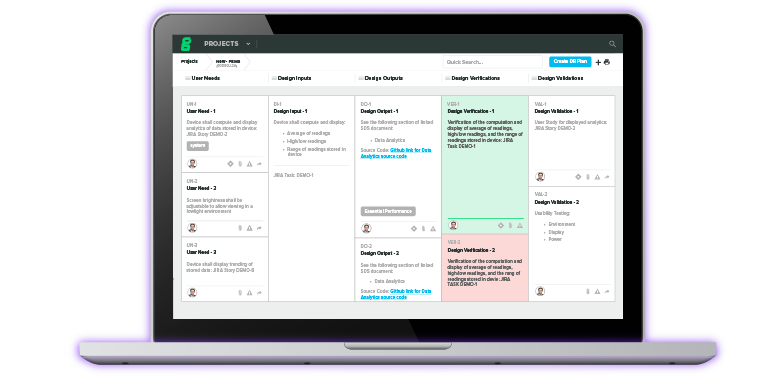
Understanding the New FDA Guidance on Changes to a 510(k)

5 Labeling Changes that Require a New 510(k)
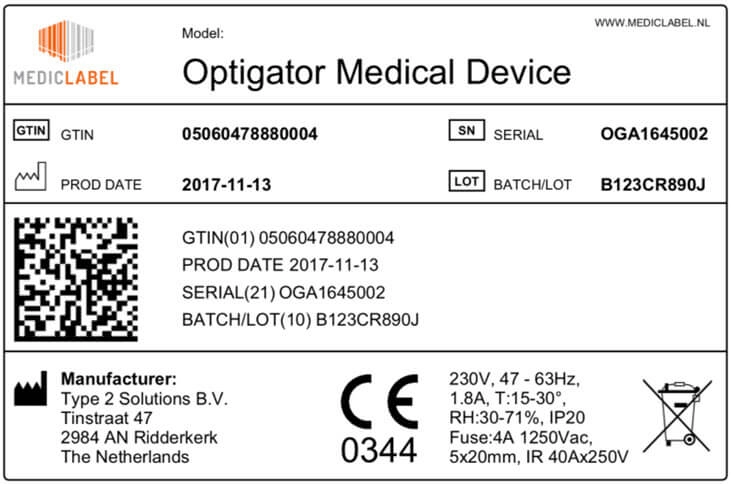
IFU for Medical Devices, a Definitive Guide (EU & US)

New Guidance from FDA: When to Submit a 510(k) for a Change to a
%20device%20software%20hardware.png?width=606&height=522&name=510(k)%20device%20software%20hardware.png)
Everything you need to know about the FDA 510(k) submission
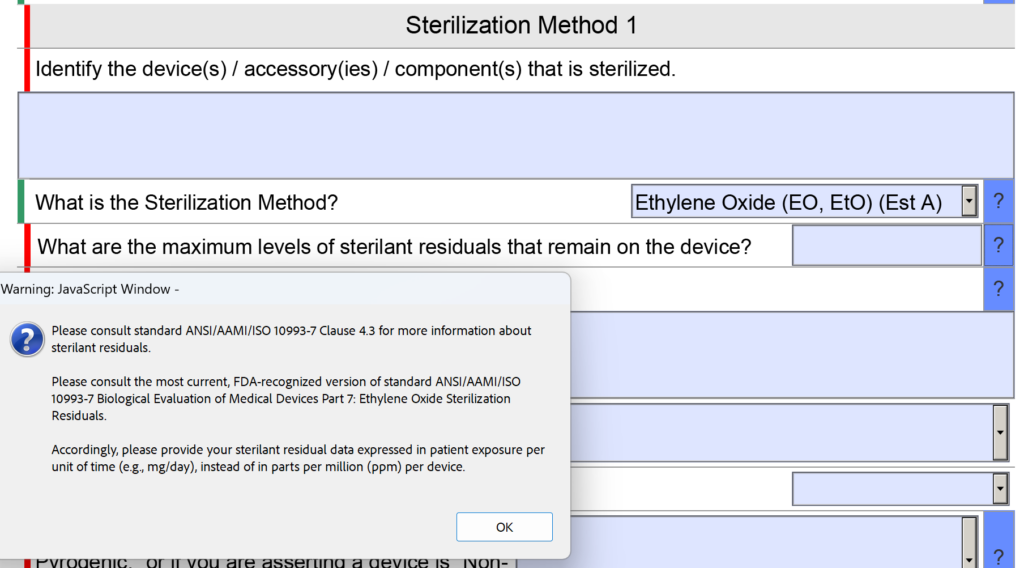
US FDA Pre-Market Notification - 510(k)
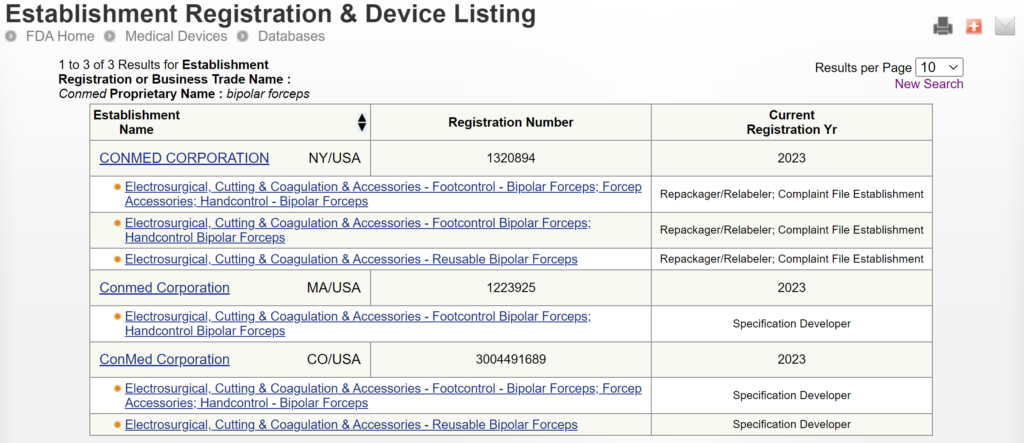
FDA
.png)
Definitive Guide to Change Management for Medical Devices
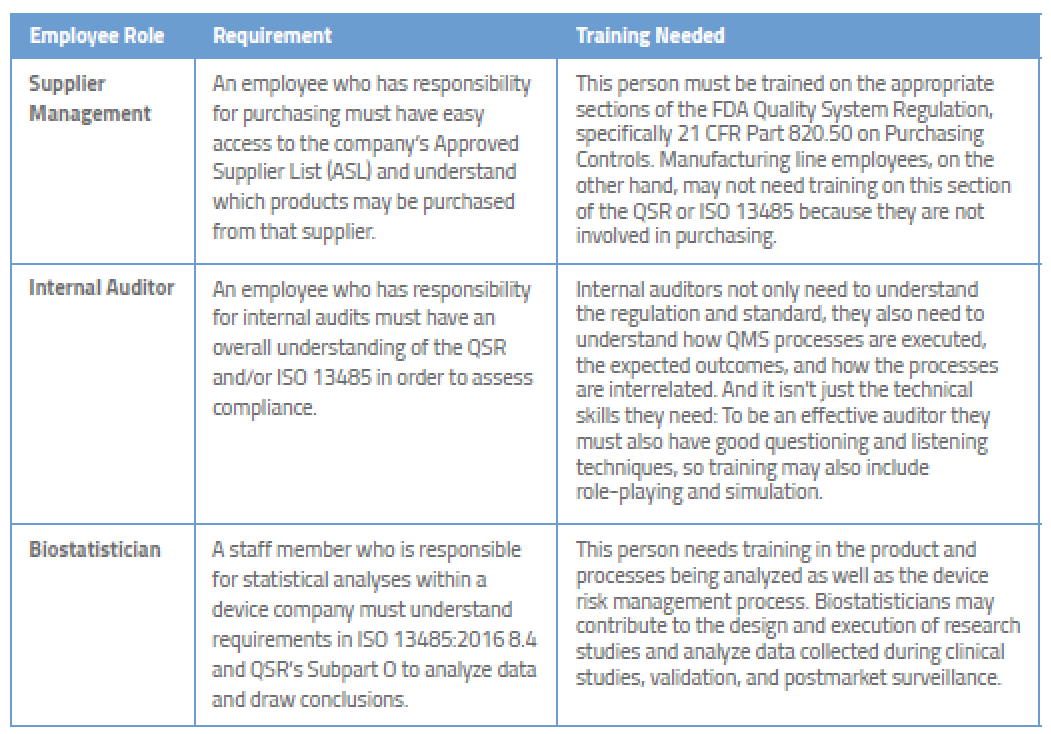
Medical Device Regulatory Training Requirements for Employees

Medical Device Changes and the 510(k) - Webinar Compliance