By A Mystery Man Writer

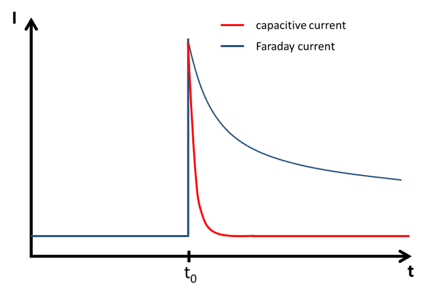
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.
The Cottrell Experiment and Diffusion Limitation 3/3
Capacitive Current - PalmSens
support/electrochemical technique
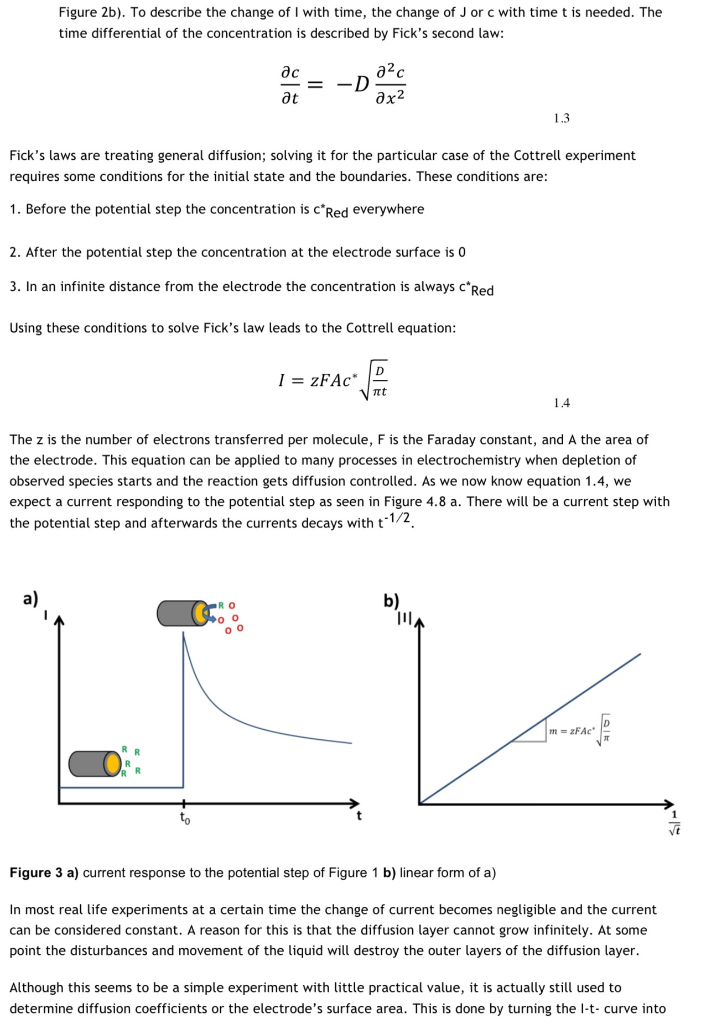
Figure 1.1: Cottrell experiment in KCl solution with

Cottrell equation - PalmSens
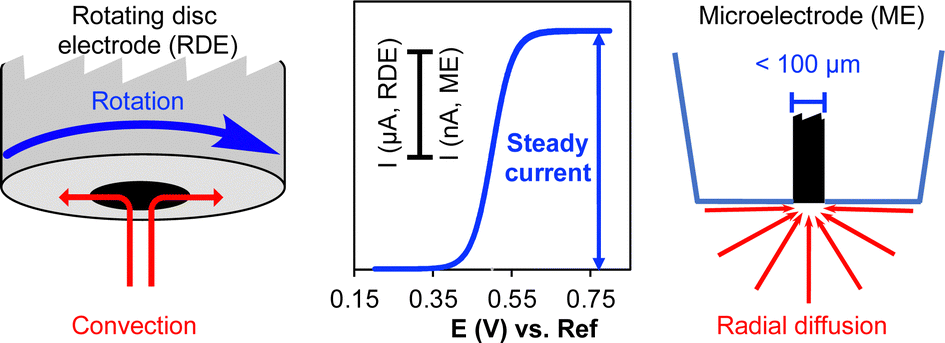
Cyclic voltammetry and chronoamperometry: mechanistic tools for organic electrosynthesis - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00706A

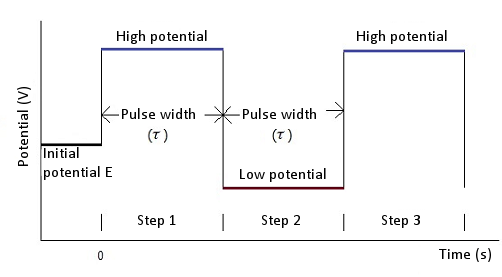
The Cottrell Experiment and Diffusion Limitation 3/3

Electrodeposition of neodymium from betaine-ethylene glycol deep eutectic solvent using neodymium oxide as a precursor - ScienceDirect
Crystals, Free Full-Text

Cottrell equation - Wikipedia

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes